Introduction. Petroleum acids have a wide range of applications in the national economy and industry. It is known that petroleum acids are abundant in the oil produced in Sabunchu, Bibiheybat, Binagadi, Balakhani and other fields of Azerbaijan. Naphthenic acids from different oil fields differ in their physical and chemical properties. In this regard, petroleum acids are of great practical importance. Petroleum acids and carbonic acids, along with diesters, are of great practical importance in both organic synthesis and petrochemical synthesis. has a wide range of applications in the fields.
Synthesis of mono diethylene glycol – and diesters on the basis of C4-C10 fatty acids in the presence of salts of petroleum acids (cobalt-, zirconium- and zirconyl) (5-6% by weight of acid) and their properties were studied [1].
Ionic liquid was synthesized in the presence of 1,4-dimethylpyperazine hydrosulfate catalyst on the basis of valerian-, kapron acids and diphenylolpropane propylene oxide monoether with divalerianate, dicapronate esters components – alcohol: acid-1,05:2 ratio, at 80-900C temperature for 5 hours . The difference between this catalyst and other catalysts is that the catalyst is taken in small amounts, which causes the reaction to be carried out under mild conditions, in a short time and without the formation of resin. Under these optimal conditions, the yield of the target product is 85-90%. Physico-chemical parameters of diesters were determined and their structures were identified by spectral methods. The synthesized diesters have been tested to improve the thermoxidation stability of diesel fuel and it has been determined that these diesters can be used as antioxidants in diesel fuel [2].
In the article [3], in the presence of nano-TiO2 catalyst (1.2% by weight of acid), natural oil acid and diphenylolpropane propylene oxide monoether in a ratio of 2:1 mol at a temperature of 110-1200C was synthesized in 14-15 hours. Physicochemical parameters of the synthesized diaphragm were determined and identified by spectral methods. The article also shows the advantages of the catalyst in the etherification process and its testing as an antioxidant for diesel fuel.
It is known from the literature [4] that glycol esters of carbonic acids are mainly offered as plasticizers and are of great interest in this regard. It is known from the literature that petroleum acids are obtained on the basis of the reaction of alkali salts of ethylene glycol esters with dichloro ethane and that they are offered as plasticizers in the manufacture of polyvinyl chloride and rubber products. The researchers showed that alkyl esters in the presence of nano TiO2 (P-25, PC-10) catalyst at 110-1500C for 3-3.5 hours in the presence of triethylene glycol, valerian, kapron, peralgon, etc. esters of carbonic acids were synthesized and tested as a plasticizer in polyvinyl chloride polymers.
In the study [5, 6] in the presence of a natural oil acid trimethylol-propane and a nano TiO2 (PC-50) catalyst, a compound of ether was synthesized at 800C with a yield of 80% for 6 hours, proposed as a plasticizer, and with dioctylphthalate industrial plasticizer. were compared. In the literature, synthetic petroleum acid was synthesized in the presence of nano TiO2 and optimal conditions for the etherification reaction were found and the material balance of the process was established.
Ethylene glycol monoethers with aliphatic unsaturated acids have been synthesized to synthesize mixed diaphragms of ethylene glycol [7]. A KU-2-8H catalyst, a hetero catalyst, was used as the catalyst, and mixed unsaturated diaphragms based on oxyethers were synthesized, which were proposed as new monomers.
The petroleum acid taken as a raw material in the submitted research work was taken from Azerneft Oil Refinery. Naphthenic acid separated from kerosene-gasoyl fraction and benzoic acid as fatty acid were used as distilled petroleum acid. Mono and bis ethers were obtained on the basis of ethylene glycol taken as a raw material. First, the purified petroleum acid was expelled under vacuum, and then synthesis reactions were carried out on its basis. Physicochemical parameters of petroleum acid used as a raw material in the study were determined and given in Table 1.
Table 1 – Physicochemical properties of distilled petroleum acid (DNA)
|
The amount of naphthenic acid, % |
Naphthenic acid acid number |
Non-soapy organic part |
ρ 420 q/sm3 |
nD20
|
Molecular weight |
|
| DNA |
98,75 |
256 |
1,25 |
0,9848 |
1,5030 |
218,75 |
Taking into account the above, the main purpose of our research was the synthesis of mono esters of natural petroleum acids and carbonic acids in the presence of ionic liquid catalysts. On the basis of synthesized mono esters, benzoic acid was used to synthesize diaphragm.
The live broadcast reaction was carried out in a three-throat flask equipped with a mechanical stirrer, a dropper funnel and a thermometer. To the flask was added 1 g/mol of petroleum acid in the amount of 10 g/mol of ethylene glycol, acid catalyst (ionic liquid N-methyl-pyrralidone hydrosulfate) in the amount of 5%, and benzene (100ml) as a solvent. The reaction was carried out at 80-900 0C for 5 hours. During the reaction, two layers are formed: the upper and the lower layer. The top layer is glycol ether of petroleum acid, and the bottom layer is the unreacted amount of ethylene glycol, which is separated from the reaction mixture by a separating funnel. After neutralizing the top layer, it was expelled in a vacuum and its physical and chemical properties were studied. Mono and bis ethers were synthesized by changing the molar content of the reactants. The etherification reaction was obtained with a yield of 80-90% for the following reaction:
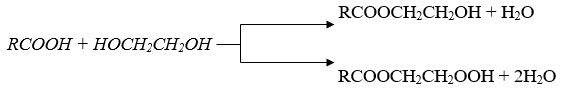
Some physicochemical parameters of synthesized mono and bis ether: boiling point of mono glycol ether of petroleum acid145-2400C; acid number 0.5 mg KOH/g, Radiation coefficient, 1.4735; density 0.9765g/cm3.
Bis glycol ether of petroleum acid boiling point 195-2950C; acid number 1.0 mg KOH/g, Radiation coefficient 1.4680; density 0.9820g/cm3.
Ionic liquid N-methyl-pyrralidone hydrosulfate was used as a catalyst in the direct ether reaction. Our goal in using this catalyst was to be environmentally non-toxic, to avoid any resin during the reaction, and to obtain ether with high yield. The catalyst was obtained according to a known method in a three-necked flask equipped with a thermometer, drip funnel, mixer and water bath. Sulfuric acid is added dropwise to the system at 00C for one hour. Then we carry out the reaction at room temperature for 24 hours. The resulting product is dried after washing the catalyst. The reaction takes N-methyl-pyrralidone and H2SO4 (98.8%) in a ratio of 1:1. The obtained catalyst was considered profitable because it can be used several times in the etherification reaction.
It has also been possible to obtain natural petroleum acid diaphragms with ethylene chloride. The experiment was carried out according to the above method, but with the participation of 40% alkali solution. The reaction was carried out according to the following scheme and was obtained with a yield of 85-90%.
In order to obtain carbon dioxide, the mono ether of carbonic acids (capron, caprylic) was carried out by the reaction with benzoic acid by the above-mentioned method:
![]()
Here R – naphthenic, kapron (C5 H11-), enant (C6H13-) radical
Physicochemical parameters of natural petroleum acid and carbonic acid monoether are given in the table.
Table 2 – Physicochemical properties of natural petroleum acid and carbonic acid monoether
|
Oxy ethyl acid esters |
Boiling point |
Exit |
Density kg / m3 |
nd20 |
Pickle number |
Soaping is numerical |
|
Naften |
140-240/2.66 |
88 |
976.3 |
1.4710 |
0.5 |
286.5 |
|
Kapron |
95-98/0.27 |
90 |
978.5 |
1.4356 |
0.8 |
362.5 |
|
Capril |
138-141/0.37 |
88 |
951.6 |
1.4401 |
0.75 |
350.6 |
The direct esterification reaction was carried out with 1.2:1 mol of benzoic acid of the reagents involved in the reaction (naphthenic acid, caproic acid, caprylic acid), soluble in toluene medium with 5% catalyst (ionic liquid N-methylpyrrolidone hydrosulfate) for benzoic acid. The etherification reaction was carried out for 5-6 hours at a temperature of 1100C. The amount of water released during the reaction was 2 g. The resulting product is neutralized with 1% triethanol amine solution, the solvent is expelled, the obtained ethers are expelled in a vacuum.
Benzioate Naftenate diaper was expelled at a pressure of 250-3300C/0.8 kPa and physico-chemical parameters were determined:; d420 = 0.960; nd20 = 1.4780; acid number 0.7 mg KOH/g, yield 86%;
Benzoate kapronate diesel; d420 = 1,044; nd20 = 1.4710; acid number 0.7 mg KOH/g, yield 85%;
Benzoate enanthate diesel; d420 = 1,031; nd20 = 1.4752; acid number 0.5 mg KOH/g, yield 84.5%;
The structures of the obtained ethers were confirmed by modern analysis methods IR and NMR. It was taken on the Bruker spectrometer by ALFA IR-Fourier. In the IR spectral analysis, 710 for the benzene ring, 1110,1173 for the C-O-C bond, 1271 cm-1, 1723 for the ether group, and the absorption band of the CH deformation and valence bond in the CH2 and CH3 groups were 1370-1450, 2850, 2927. In addition, in the CH2 group close to the CO group, the absorption band of the CH bond was 1410 cm-1, for the CO group C = O 1700 cm-1, for the C-O 1170 and 1230 cm-1.
Chemical slip signals for CH2 and CH3 groups according to NMR analysis
δ = 0.88-1.3m.h., characteristic for naphthene hydrocarbon protons 1.8-2m.h., signal for methylene protons 3.27-3.37m.h. was observed.
The synthesized diesters , including naphthenic acid monoethylene glycol benzoyl ether, were tested in PVC (polyvinyl chloride) resin as a plasticizer and compared with the standard dioctylphthalate (DOF) and confirmed to be replaceable. The direct esterification reaction was carried out in the presence of a mono, bisester ionic liquid catalyst of petroleum acid. N-methylpyrrolidone hydrosulfate was used as the catalyst for the ionic liquid. The catalyst is environmentally friendly and more cost-effective as it can be reused. Due to the short etherization time, no resin product was observed during the reaction and it was synthesized with high yield. The mono, bis ether of the synthesized petroleum acid has been proposed as a plasticizer due to its superior properties compared to the industrial catalyst.
References
- Iskenderova S.A., Sadieva N.F., Zeynalov E.B. and others. Development of a method for the synthesis of mono – and symmetric diethylene glycol esters of fatty acids // Processes of Petrochemistry and Oil Refining, 2006, №3 (26), p.3-8.
- Karimov P.M., Eleskerova U.M., Nuriyev L.H. and b. Synthesis and application of diefirs on the basis of monoephyr of propylene oxide of valerian, capronic acids and diphenylolpropane with the participation of Ion liquid 1.4-dimethylpiperazindihidrosulfate catalyst // Azerbaijan oil Economy, 2017, № 1, p.33-35.
- Zeynalov E.B., Karimov P.M., Aliyeva S.Q. and others. Synthesis of diefir on the basis of monoephyr of propylene oxide of Natural Petroleum acid and diphenylolpropane with the participation of nano-TiO2 (PC-500) catalyst and study of its antioxidant properties // “Scientific Works” Journal of AMU, 2014, №2, Vol.2, p.283-287.
- Zeynalov E.B., Karimov P.M., Aliyeva S.Q., Eleskerova U.M. Agayev B.K., Salmanova C.Q., Guliyeva E.M. Synthesis of diefirin and its antioxidant properties on the basis of monoephyr of propylene oxide of Natural Petroleum and diphenylolpropane with the participation of nano-TiO2 (PJ-500) catalyst. Azerbaijan Technical University, Baku, scientific works, 2014, № 2, p.283-387.
- Application 10104597 (Germany). Antithrombotic derivatives, their preparation and use as medicines. Ries Uwe, Prierke H. Publ. in RZHHIM., 2003, 03. p-190.81 p.
- Application 1350790 (Japan). Method of obtaining active esters. Okuyama S., Masako H. Publ. in RZHHIM., 2004, 04.06-190.1643 p.
- Mustafaev S.A., Veliyev M.G., Shakhmamedova A.G. Synthesis and study of antimicrobial activity of marginal and unsaturated esters of petroleum naphthenic and cyclohexenecarboxylic acids. Azerbaijan Chemical Journal, Baku-2014, №2, pp.22-25.

 View this article in Russian
View this article in Russian