The purification of the oil feedstock selective solvent plays an important role in modern integrated circuit refinery. The use of selective solvents significantly expand the resources of oil and raw materials to include in the processing of heavy oil to the resinous oil, oil distillates, which are difficult to clean with sulfuric acid. Due to the need for an economically viable process and a selective treatment in order to obtain high quality oils for selective solvents are strict and diverse requirements. Therefore, a huge number of studied and proposed selective solvents for cleaning oil fractions found practical application, only a few of them. Among the few polling solvents include phenol. The selective purification oil with phenol is the most common process in the CIS countries and abroad. By classical concepts phenol as a selective solvent, in contrast to furfural characterized by high dissolving power and an average selectivity, whereas furfural is highly selective and of low solubility. However, recent studies have refuted these ideas. Phenol has a highly selective with respect to the components of the group of oils, but inferior furfural selectivity for their aromatic part. Therefore, when cleaning the oil feedstock selective solvents of the paraffinic base oil should be preferred phenol. Although high selectivity to phenol group components oil extracts obtained by phenol purification contain significant amounts of oil components, which is due to the high solubility of phenol [1, p. 152].
The extracts after purification distillate feed contains 20-30% of component a viscosity index viscosity of 60 to 115. The chemical composition of the study group distillate extracts demonstrated that these extracts contain between 25% and 34% of paraffin-naphthenic hydrocarbons. By fractionation of distillate extracts phenol has been allocated about 40% of the secondary raffinate, the quality of which was close to the quality of the relevant oil distillates [2, p. 68].
The aim of this work is to study the physical and chemical properties of oil distillates Kumkol oil and phenol by selective purification, isolation from extracts of the secondary oil distillates that meet the requirements of interplant standards in the relevant product of high viscosity oil distillate.
Research Kumkol oil distillates of crude oil (Table 1) showed that these fractions are little gummy and contain significant amounts of 72.5% and 78.3% paraffin-naphthenic hydrocarbons. Physico-chemical properties of the group and the chemical composition of the samples studied oil distillates is in close relationship with their fractional composition.
Thus, the second oil distillate fraction 420-480 °C as compared with the third oil fraction 480-500 °C epaulet has smaller values of density, kinematic viscosity, refractive index, pour point and average molecular weight.
Higher values of indicators such as refractive index, viscosity and weight constant, aniline point and unit variance for a third of the oil distillate indicate a higher degree of its aromatization, as evidenced by research group of chemical compositions of both products.
Table 1 – Physical-chemical characteristics of oil distillates Kumkol petroleum
| Thenameofindicators |
2nd oil strap fraction 420-480 °C |
3rd oil strap fraction 480-500 °C |
|
| Density |
0.8851 |
0.9016 |
|
| Refractioncoefficient |
1.4780 |
1.4850 |
|
| Viscosity, cSt |
υ50 |
14.9 |
36.7 |
|
υ100 |
4.1 |
7.4 |
|
| Viscosity-weightconstant |
0.837 |
0.849 |
|
| Intertseptrefraction |
1.0475 |
1.0462 |
|
| Congelationpoint, оС |
27.5 |
35.0 |
|
| Averagemolecularweight |
340 |
410 |
|
| Specificdispersion |
140 |
151 |
|
| Anilinepoint, оС |
90 |
97 |
|
| Groupchemicalcomposition, % | Paraffin-naphthenichydrocarbons |
78.3 |
72.3 |
| Lightaromatichydrocarbons |
8.8 |
9.9 |
|
| Averagearomatichydrocarbons |
5.5 |
6.2 |
|
| Heavyaromatichydrocarbons |
6.0 |
8.5 |
|
| Resins |
1.4 |
2.9 |
|
| Structuralgroupcomposition, % |
СР |
59 |
53.5 |
|
СN |
30 |
35.5 |
|
|
СА |
11 |
11 |
|
| Viscosityindex |
– |
83 |
|
| Flash-point, 0С |
– |
206 |
|
At the same time, analysis of the distribution of carbon in the samples indicates that the carbon number constituting the aromatic structures of both products alike. However, a larger amount of carbon constituting the third structures naphthenic distillate oil indicates the presence of a larger amount of naphthenic hydrocarbons with a high refractive index of the group of the chemical composition.
Clearly, the study of structural-group composition can more accurately characterize the chemical nature of the oil distillates and can serve as the main indicator when compared with the secondary raffinate obtained by selective re-clean distillate extracts.
In order to clarify the fundamental possibility of allocating secondary raffinate from Kumkol oil distillate extracts, the latter were subjected to physical and chemical research, the results of which are shown in Table 2.
As shown by the data in Table 2 extract obtained by selective purification of phenol second oil distillate (extract 2nd distillate), in spite of the lighter fraction composition is a flavored product, than the extract obtained by selective purification of phenol third oil distillate (extract 3 th distillate).
Table 2
| Thenameofindicators |
Extract 2nd distillate |
Extract 3rd distillate |
||
| Density |
0.9949 |
0.9506 |
||
| Refractioncoefficient |
1.5560 |
1.5170 |
||
| Viscosity, cSt |
υ50 |
72.2 |
94.5 |
|
|
υ100 |
8.3 |
11.3 |
||
| Viscosity-weightconstant |
0.962 |
0.904 |
||
| Intertseptrefraction |
1.0705 |
1.0537 |
||
| Congelationpoint, оС |
1 |
30 |
||
| Averagemolecularweight |
300 |
373 |
||
| Specificdispersion |
229 |
167 |
||
| Anilinepoint, оС |
17 |
68 |
||
| Groupchemicalcomposition, % | Paraffin-naphthenichydrocarbons |
10 |
41 |
|
| Lightaromatichydrocarbons |
8 |
14 |
||
| Averagearomatichydrocarbons |
29 |
13 |
||
| Heavyaromatichydrocarbons |
45 |
27 |
||
| Resins |
8 |
5 |
||
| Structuralgroupcomposition, % |
СР |
34 |
37 |
|
|
СN |
24 |
36 |
||
|
СА |
42 |
27 |
||
Thus, at lower values of kinematic viscosity and molecular weight of the extract of the second shoulder strap has a significantly high values of indicators such as density, refractive index, viscosity and weight constant intertsept refraction and dispersion of specific than extract 3rd distillate.
Greater aromatic extract 2nd distillate supported by research group the chemical and structural composition of the group. Thus, compared with an extract of the third distillate extract 2nd distillate contains significantly less paraffin-naphthen hydrocarbons (41% and 10%) more heavy aromatics (27% and 45%) and resin (5% and 8% respectively).
Furthermore, the amount of carbon constituting the aromatic structures 2nd extract distillate is significantly larger than the carbon number constituting the aromatic structures 3rd extract distillate of 42% and 27% respectively.
Thus, studies have shown that an extract of the 3rd distillate contains significant amounts of valuable components and oils can serve as the raw material for the isolation of the secondary raffinate, meeting the requirements of interplant standards impose an oil distillate.
As for the 2nd extract distillate, the content of valuable oil components are not significant and the selection of his recycled raffinate present considerable technical difficulties, low critical solution temperature that is unlikely to be economically feasible.
Isolation of the secondary extract from the raffinate third distillate carried by repeated selective treatment of the latter. To carry out this cleaning in industrial conditions necessary to establish the optimal parameters of a technological mode extraction column corresponding to the highest yield of secondary raffinate required qualities. To this end, we studied the effect of various factors (temperature, to a multiplicity of raw phenol, the amount of water supplied to the extraction) on the yield and quality of the secondary raffinate by a single treatment 3rd extract distillate phenol.
To determine the optimum ratio of solvent to raw material, as well as the curves of the CTE of raw materials and solvents used for the preliminary determination of the extraction temperature in accordance with Figure 1.
As can be seen from the graph according to Figure 1, an optimal ratio of solvent to raw materials is 3:1. To reduce the solvent power of phenol and accordingly increase its compatibility, we studied the effect of various additives to the solvent amounts of water on the CTE value.
By adding 7% of water to a solvent with its CTE feedstock was 116 °C, which will carry out the extraction at temperatures upper extraction columns, normally used in industry (85-95 °C) as the extraction temperature is normally used at 15-20 °C below the maximum value CTE.
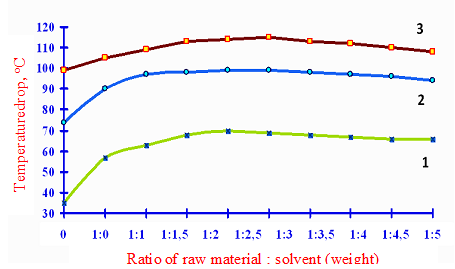
1 – dry phenol; 2 – phenolate + 5% water; 3 – phenol + 7% water
Figure 1 – The dependence of CTE on the amount of solvent to raw materials multiplicity and extent of flooding phenol
Processing the extract with phenol was carried out in a glass batch extractor provided with a stirrer for an intensive stirring. The extraction temperature was maintained thermostated circulating liquid (water) through the jacket of the extract. In all experiments, the extraction time was 30 min. settling time 45 minutes. After the sludge extraction solution blended through the bottom valve and discarded, and the solution was going to raffinateWurtz flask through a tube. After regeneration of the solvent distilled under vacuum at a temperature not higher than 1800оС determined raffinate yield (% by weight on the feedstock) and its kinematic viscosity at 1000оС. Conditions and results of a single treatment with phenol extract 3rd distillate shown in Tab.3 and in Fig. 2-4.
Table 3 – Conditions and results of a single treatment with phenol extract of the third distillate
| № |
Extractiontemperature, |
Amount of phenol, % of raw materials |
Amount of water, % to phenol |
Output of raffinate, % of raw materials |
Kinematicviscosityυ100, cSt |
|
| 1 series |
1 |
70 |
200 |
10 |
55.4 |
9.3 |
|
2 |
80 |
200 |
10 |
62.5 |
9.4 |
|
|
3 |
85 |
200 |
10 |
64.4 |
9.5 |
|
| 2 series |
4 |
70 |
300 |
10 |
56.7 |
9.2 |
|
5 |
80 |
300 |
10 |
54.1 |
9.1 |
|
|
6 |
90 |
300 |
10 |
50.7 |
9.1 |
|
| 3 series |
7 |
90 |
300 |
10 |
50.7 |
9.1 |
|
8 |
90 |
300 |
7 |
37.7 |
8.8 |
|
|
9 |
90 |
300 |
5 |
23.7 |
8.5 |
|
As the data in Tab.3 and in accordance with Fig. 2-4 at a constant multiple of raw phenol (2: 1) and the same quantity of water supplied to the extraction (10% phenol) increasing the extraction temperature from 70 °C to 85 °C It increases the yield of secondary raffinate and viscosity, and the viscosity greatly exceeds raffinate interplant standards requirements (7.0-8.5 cSt.).
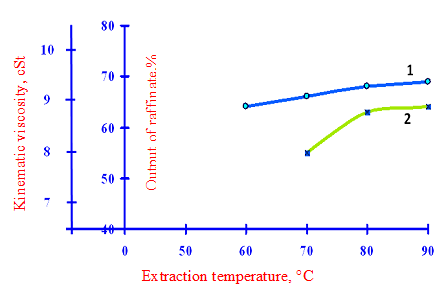
1 – kinematic viscosity secondary raffinate; 2 – the output of the secondary raffinate
Figure 2 – The effect of temperature on the extraction yield and the quality in the secondary raffinate to a multiplicity feedstock phenol – 2:1 and the amount of water supplied to the extraction of 10%
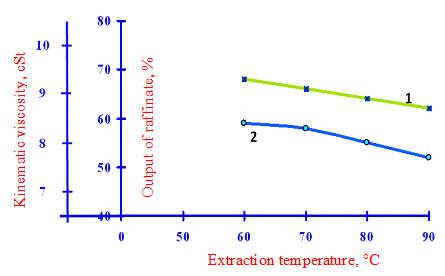
1 – Kinematic viscosity secondary raffinate; 2 – the output of the secondary raffinate
Figure 3 – Effect of extraction temperature on the yield and quality of the phenol in the secondary raffinate to a multiplicity of feed – 3: 1 and the amount of water supplied to the extraction of 10%
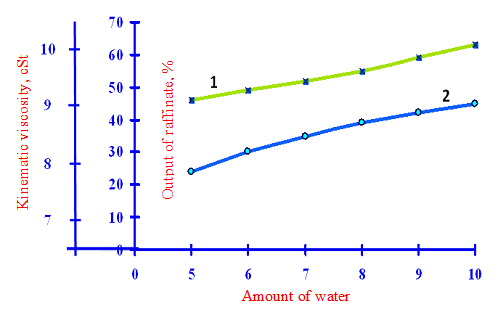
1 – kinematic viscosity secondary raffinate; 2 – the output of the secondary raffinate
Figure 4 – Effect of the amount of water supplied to the extraction, the yield and the quality in the secondary raffinate to a multiplicity feedstock phenol – 3:1 and the extraction temperature – 90oC
Increasing multiplicity of phenol to the feed up to 3:1 in the same conditions (Tab.3, in accordance with Fig.4) led to some reduction in viscosity and the yield of secondary raffinate, but the latter varies only slightly and is still high. The biggest impact on the yield and quality of the secondary raffinate having the amount of water supplied to the extraction. Thus, the extraction at a temperature of 90 °C and a feedstock phenol multiplicity 3:1 reduction in the amount of water supplied to the extraction with 10% to 5% of viscosity leads to a decrease from 9.1 cSt to 8.5 cSt and exit from 50.7% to 23.7%.
Based on the above pre-conditions for the optimal allocation of the secondary extract from the raffinate third distillate adopted:
a) Extraction temperature °C – 90;
b) Multiplicity of raw phenol (weight) – 3:1;
c) The amount of water supplied for extraction,% (wt) to phenol – 7.
A single raw processing phenol can not simulate the extraction of industrial machines, and only allows to determine the temperature of the column top. However, on top of a known temperature, taking into account the temperature gradient extraction (10-25 °C), you can simulate the industrial extraction column by means of the three-stage countercurrent extraction periodic.
References
- Omaraliev T.O. The special technology of fuel production from oil and gas. -Astana.: Foliant, 2005.- 294 p.
- Akhmetov S.A.Chemistry and technology of deep processing of oil and gas. – Ufa.: Bilim, 2002.- 249 p.
- Chernozhukov N.I. Oil and gas processing technology. Part 3. – M.: Chemistry, 1978. – 424 p.
- Nadirov N.K. Oil and Gas of Kazakhstan. Part2. – Almaty.: Gylym, 1995. – 400 p.
- Karabaev J.A., Kapustin V.M., Tanashev S.T., SakibaevaS.A.and others. The intensification of the process of vacuum distillation Kumkol oil fuel oil by adjusting the phase transition oil disperse systems.CHEMISTRY AND TECHNOLOGY OF FUELS OILS Volume: 577 Issue: 3 Pages: 33-37 Published: DEC 2013
- Sunyaev Z.I. Physico-chemical mechanics of oil disperse systems. – M.:Gubkin I.M., 1981. – 91 p.

 View this article in Russian
View this article in Russian