There are two well known ways of generating energy, using salt and fresh water mixing.
First of them is mechanical way, when a vessels with a salt and fresh water are separated by a semi-permeable membrane. The disadvantage of this way is high energy loss during electricity generation, because electric power generates in two stages.
The second one uses ion selective membranes. In this case electric power generates in one stage. The disadvantage of second way is a high resistance of ion-selective membranes.
We develop the third way of converting entropy of fresh and salt water mixing into electrical energy, using concentration galvanic cell.
Of course NaCl solution is not the best working solution for concentration galvanic cell. We may use Ag/AgCl electrode, but they are too expensive for high-scale generation.
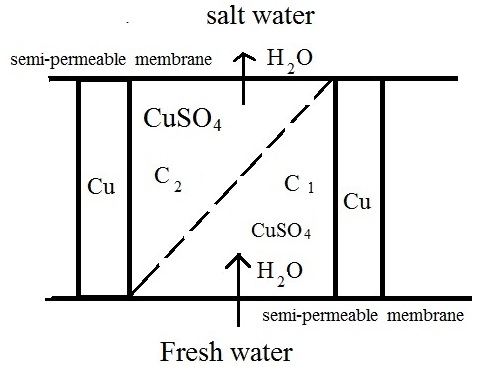
So we have such a scheme of our cell:
salt water || semi-permeable membrane || working solution of high concentration|| porous diaphragm ||working solution of low concentration || semi-permeable membrane || fresh water.
Figure 1. Concentration galvanic cell
In the picture: C2>C1
The osmotic pressure at the working solution of low concentration is higher, then osmotic pressure at fresh water, so the water passes through the semi-permeable membrane into concentration galvanic cell and maintains a low concentration of the electrolyte near the first electrode.
The osmotic pressure at the salt water is higher, then osmotic pressure at working solution of high concentration, so the water passes through the semi-permeable membrane out from concentration galvanic cell and it maintains a high concentration of the electrolyte near the second electrode.
So, NaCl solution never takes part at the reaction, but absorbs water from working solution of high concentration!
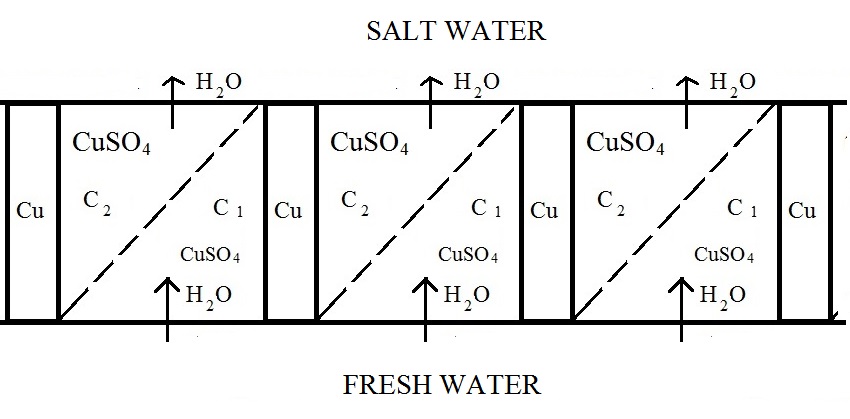
Elements from Figure 1 could be connected in a cascade:
Also interesting results could be obtained by using sulfuric acid as working solution and Pb/PbSO4 electrodes.
In contrast to the traditional concentration galvanic cells, where the potential difference depends of the lg c1/c2, in sulfuric acid solution we have practical linear dependence of the electrode potential on the concentration of sulfuric acid. We have this effect because galvanic cell uses not only entropy factor, but also the energy of exothermic reaction of sulfuric acid dissolving in the water.
References
- Вассель С.С., Вассель Н.П. ИСПОЛЬЗОВАНИЕ НИЗКОПОТЕНЦИАЛЬНОЙ ТЕПЛОТЫ В ЭЛЕКТРОХИМИЧЕСКОМ ПРЕОБРАЗОВАТЕЛЕ. Современные научные исследования и инновации. 2014. № 4 (36). С. 21.

 View this article in Russian
View this article in Russian