Traditionally thermogalvanic cells are consists of two similar metal electrodes, immersed into vessels with the solutions of their salts. This vessels has different temperatures, and it is the reason of appearance of the potential difference between electrodes. But thermogalvanic cells of such construction are not such effective as semiconductor thermocouples[1].
In our devise we decide to use the reaction of sulfuric acid or sodium solvation. This reaction is reversible over a high temperature range. It gives us the possibility to increase the efficiency of thermogalvanic cells greatly.
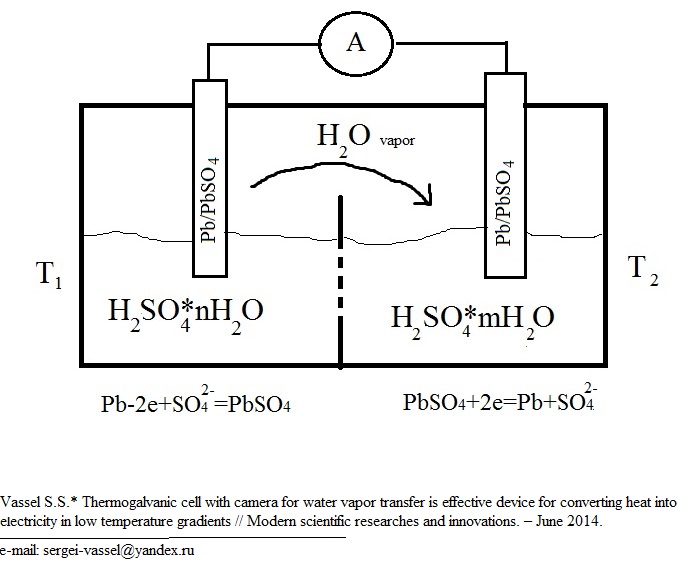
The construction of our chemical thermocouple is presented in Fig.1.
Fig. 1. The sheme of thermogalvanic cell with camera for water vapor transfer.
As we can see, the construction of our device is more complex, then the construction of traditional thermogalvanic cells, because hot and cold vessels are also connected by vacuum camera for water vapor transfer. So the work of thermogalvanic cell with water vapor transfer cold be devided into 2 processes: distillation of sulfuric acid solution in temperature gradient and generating electrical energy in galvanic cell.
To predict the possibility of the distilling we must have an information about the vapor pressure above the solutions. The information of vapor pressure above the sulfuric acid solutions (pesented at Table 1) we take from sourse[2].
Table 1. The total vapor pressure (in mm Hg. Cent.) of sulfuric acid solutions at different temperatures
| c,% |
10 ° C |
20 ° C |
30 ° C |
40 ° C |
60 ° C |
80 ° C |
100 ° C |
|
10 |
8.80 |
16.60 |
30.2 |
52.7 |
141.1 |
337 |
723 |
|
20 |
8.20 |
15.21 |
27.8 |
48.4 |
130.0 |
312 |
668 |
|
30 |
6.75 |
12.73 |
23.1 |
40.7 |
111.7 |
273 |
593 |
|
40 |
4.95 |
9.51 |
17.26 |
30.8 |
86.1 |
218 |
488 |
|
50 |
2.95 |
5.95 |
11.18 |
19.91 |
58.4 |
147.2 |
338 |
|
60 |
1.387 |
2.80 |
5.30 |
9.70 |
29.1 |
76.4 |
175.0 |
|
70 |
0.400 |
0.827 |
1.653 |
3.13 |
9.65 |
26.1 |
63.9 |
|
80 |
0.057 |
0.116 |
0.219 |
0.397 |
1.398 |
5.00 |
14.52 |
|
85 |
0.018 |
0.042 |
0.188 |
0.188 |
0.636 |
1.95 |
6.15 |
In Table 1 “c” is the concentration of H2SO4,% (by weight)
For example, the vapor pressure above the 50% solution of H2SO4 at 40 C is higher, then above 10% solution at 20C. So, if the temperature of hot contact is 40° C, and the temperature of cold contact is 20° C, we can perform the distillation of sulfuric acid from 10% to 50% solution.
The potential of Pb/PbSO4 electrode in sulfuric acid solution depends on it the concentration.
The electrode process at the vessel with high concentration of H2SO4 is:
Pb+SO42- —2e→PbSO4
The electrode process at the vessel with low concentration of H2SO4 is:
PbSO4+2e→Pb+SO42-
In traditional concentration galvanic cells, which use only entropy factor, the potential difference depends on concebtration as lgc1/c2. In sulfuric acid solutions we have practical linear dependence of the electrode potential on the concentration of sulfuric acid. We have this effect because galvanic cell uses an energy of exothermic reaction of sulfuric acid dissolving in the water.
The empirical formula of Pb/PbSO4 electrode potential is E = E0 + p / 2 [3], where E0=const and ‘p’ is the density of sulfuric acid in g/cm3. This formula is valid for sulfuric acid concentrations from 0 to 60%. The electromotive force (EMF) of concentration cell consisting of two Pb/PbSO4 electrodes immersed in sulfuric acid solutions of different concentrations is described by formula EMF = Δp /2 The potential difference between the lead electrode in 10% sulfuric acid solution, and lead electrode in 50% sulfuric acid solution of sulfuric acid is 0.165 V (165 mV), which corresponds to 8 mV/K.
The efficiency of the device depends not only on the temperature difference, but also on the initial and final acid concentration. More infomation about this effect you ca find in paper [4].
The maximum EMF and efficiency of chemical thermocouple is presented at Table 2.
Table 2 The maximum EMF and efficiency of chemical thermocouple at different temperatures.
|
Temperature of hot side, [C] |
Temperature of cold side, [C] |
Concentration C1,[ %] |
Concentration C2, [%] |
EMF , [V] |
Efficiency, [%] |
|
40 |
20 |
10 |
50 |
0.16 |
0.9 |
|
40 |
20 |
40 |
55 |
0.11 |
4.6 |
|
40 |
20 |
50 |
60 |
0.08 |
5.3 |
|
60 |
20 |
10 |
60 |
0.22 |
1.3 |
|
60 |
20 |
40 |
65 |
0.16 |
8.3 |
|
60 |
20 |
50 |
70 |
0.14 |
10.4 |
|
80 |
20 |
10 |
73 |
0.3 |
1.7 |
|
80 |
20 |
67 |
75 |
0.08 |
10 |
|
80 |
40 |
65 |
75 |
0.08 |
9.7 |
As we can see, chemical thermocouples could be effective in electricity generation, using little temperature differences. We can get an efficiency, rather close to the efficiency of the Carnot cycle using chemical thermocouples under certain conditions. Such efficiency make it possible to use such chemical thermocouples in external combustion engines, in hybrid cars for using the waste heat of the exhaust gases, and for using solar and geothermal heat.
References
- T. Quickenden and Y. Mua, J. Electrochem. Soc. 142, 3985-3994 (1995)
- V. Rabinovich, Z. Havin, A brief chemical handbook (Chemistry, Leningrad, 1978), p. 285.
- M. Dasoyan, Starter batteries: The device, operation, maintenance (Transportation, Moscow, 1991), p. 142.
- Вассель С.С., Вассель Н.П. Использование низкопотенциальной теплоты в электрохимическом преобразователе // Современные научные исследования и инновации. – Апрель 2014. – № 4 [Электронный ресурс]. URL: http://web.snauka.ru/issues/2014/04/33948 (дата обращения: 26.05.2014).